The various positions taken by health authorities and bodies are now in favor of the digitization of clinical trials. A pilot phase has just been launched, in which healthcare industry sponsors have an important role to play. This is an opportunity not to be missed!
The digitization of clinical trials is a major challenge in Europe, and more specifically in France. There are two main reasons for this: to maintain the attractiveness of countries as clinical research operators, and to anticipate potential new health crises.
Digitization is a generic term used to refer to dematerialization and decentralization. "Dematerialization" refers to the transformation of processes and documents from paper to digital format, such as the use of electronic signatures or remote monitoring. "Decentralization" means conducting clinical trials outside of traditional research locations, with the implementation of tools or intermediaries. Examples include the involvement of healthcare providers who visit patients' homes or teleconsultation.
Digitization is a necessary step toward decentralization.
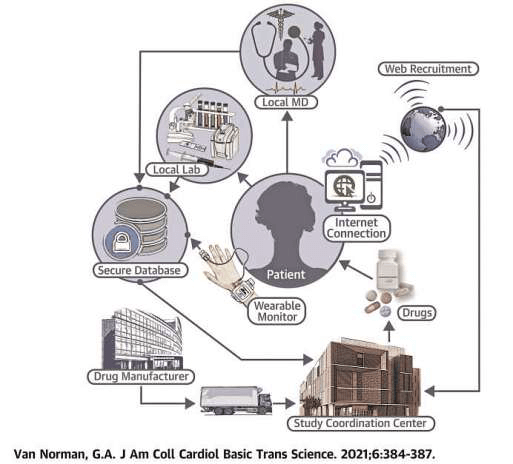
Diagram of the decentralized clinical trial process
In decentralized clinical trials, patient visits, interactions with healthcare providers, and laboratory tests are conducted as close as possible to the patient's place of residence. The products under investigation (medicines, medical devices, etc.) are supplied directly to the patient or to the local healthcare facility. Other interactions, includingpatient recruitment and follow-up, are conducted via the web with secure data access and storage. The term "wearable" used in this figure refers to all objects—clothing or accessories—that are connected to a device, such as a phone, to collect data about the person wearing them and their environment (e.g., smartwatches, connected dentures for athletes, etc.).
In the wake of the pandemic...
In March 2020, the European Commission, the European Medicines Agency, and the Heads of Medicines Agencies(HMA) published recommendations for clinical trial sponsors on how to manage the conduct of these trials in light of the new challenges posed by the COVID-19 pandemic (1). The aim of these recommendations was twofold: to preserve the quality of the data collected and to guarantee the rights, safety, and well-being of patients participating in trials.
One paragraph was devoted to remote source data verification (rSDV). It specified that this monitoring method could be considered on an exceptional basis and for certain justified situations. This rSDV should focus on quality control of critical data such as primary efficacy data and important safety data.
In April 2021, still in the context of the health crisis, the CNIL issued provisional recommendations for remote monitoring (2). Until December 31, 2021, it was possible to conduct rSDV for a limited scope of trials covered by the exemption: studies related to COVID-19, trials involving serious diseases, trials for which the absence of control of source documents was likely to pose risks to the safety of participants, etc. The scope described as limited in the CNIL's recommendations was in fact quite broad and enabled many sponsors to guarantee patient safety and continue "pivotal" clinical trials, so as not to delay the marketing authorization application or jeopardize the reliability and integrity of the results of such trials.
Interestingly, in terms of regulations, the CNIL stated the following: "...all studies initiated on or after January 1, 2022, involving remote quality control will require, as of that date, the submission of an authorization request to the CNIL. ... Data controllers who have already begun their studies may continue to use these methods beyond December 31, 2021, without submitting an authorization request to the CNIL.
In other words, those who had started rSDV with the initiation of a study in 2021 were allowed to continue, but this monitoring method required an authorization request for subsequent studies. Double standards...
New recommendations applicable outside of health crises
In December 2022, the European Commission published recommendations on decentralized clinical trials (3). The COVID-19 pandemic had highlighted the importance of digital tools and the need for decentralization procedures in healthcare and research. It was necessary to formulate new recommendations for conducting decentralized clinical trials, regardless of any health crisis.
The aim of these recommendations is to facilitate the use of decentralized components in clinical trials, while maintaining a high level of safety for participants, ensuring the protection of their rights, and guaranteeing the reliability of the data to be collected and processed. This document considers various aspects of decentralized trials, including the monitoring of clinical trials, the roles and responsibilities of the sponsor and investigators, procedures at the patient's home related to the trials, electronic informed consent, home delivery and administration of drugs, and finally the definition and processing of source data.
20 decentralized clinical trial projects soon to be studied
In February 2023, the National Commission for Research Involving Human Subjects (CNRIPH) organized a working group bringing together regulatory representatives, operators, and users to transpose these European recommendations at the national level. The six components of decentralized clinical trials listed by the European Commission are being examined in order to establish specific and concrete national recommendations, remove regulatory barriers, and promote this new form of research. This work is currently being consolidated.
Pending the French recommendations implementing the EMA (European Medicines Agency) proposals on this subject, the Ministry of Health is looking for use cases in France to further the work already underway. In January 2024, in line with these European recommendations, the Ministry of Health, through the Directorate General for Health (DGS) and the Directorate General for Healthcare Provision (DGOS), the National Agency for Medicines Safety (ANSM) and the CNIL launched a pilot phase to provide concrete answers to the difficulties encountered with one or more decentralized elements during the design of a clinical trial.
This six-month pilot phase aims to select 20 projects to receive special support. Each application must include a complete implementation plan for the decentralized element of the research project. The objective of this pilot phase is to publish French recommendations on decentralization and will contribute to the CNIL's work on updating reference methodologies.
All players in accelerated digitalization!
The path to modernizing clinical trials through digitalization is now open in France!
The pilot phase is an opportunity for any industrial or academic sponsor considering conducting a study involving a drug, medical device, in vitro diagnostic medical device, or human subjects.
This applies to:
- pre- and post-marketing clinical trials,
- performance studies,
- and clinical investigations prior to and after CE marking.
Regardless of the digital tool or process involved (see Figure 1), it is possible to submit a query to the one-stop shop with the guarantee of receiving a response within 15 days (5).
Now is the time to integrate rSDV with a secure web platform that meets the required security criteria (encryption of health data, dual authentication access, etc.) to ensure compliance with the Public Health Code and the provisions of the General Data Protection Regulation.
Contributing to the recognition and regulation ofthe digitization of clinical trialsis now a civic duty, in both an ethical and responsible sense.
Ethical, because this research method allows all patients and investigators, regardless of their geographical location, to participate in research. Responsible, because it limits the need for travel, which has a positive impact onthe carbon footprintassociated with clinical research. It is important to remember that this digitalization must above all be done in the service of the patient. The quality of the relationship between the research team and the research participant is the key to the successful evolution of practices.
Frequently Asked Questions
Need assistance?
Are you considering incorporating decentralized components into your clinical trial? Our experts CLINACT will assist you with methodological design, setting up the rSDV, and remote monitoring of your study.
1 Guidance on the Management of Clinical Trials during the COVID-19 (Coronavirus) pandemic
https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf. Accessed February 13, 2024.
2 https://www.cnil.fr/fr/recommandations-provisoires-controle-qualite-essais-cliniques-crise-sanitaire
3 HMA – EMA: Recommendation Paper on Decentralized elements in clinical trials – version 01, December 13, 2022
4 https://www.cnil.fr/fr/essais-cliniques-decentralises-lancement-dune-phase-pilote-par-la-dgs-la-dgos-lansm-et-la-cnil
5 phasepiloteessaisdecentralises@sante.gouv.fr



