A common architecture in many clinical investigations, the master protocol is beginning to be used in the medical device sector. In this article, the MultiHealth Group explains what it is, and highlights the benefits that developers can derive from it.
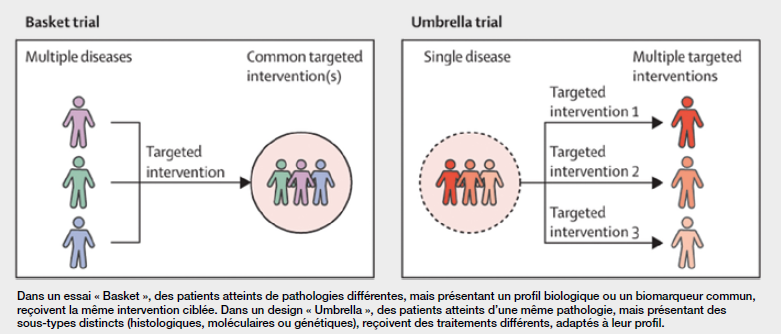
Historically reserved for drug treatments in oncology, the master protocol is now being explored in other fields, notably medical devices. The design of clinical investigations based on master protocol aims to increase efficiency in terms of trial infrastructure and protocol management, and to optimize the development of technical and technological innovations. The master protocol concept came into its own in the 2010s with the development of innovative designs such as basket, umbrella (see Figure) and platform studies. These adaptive designs make it possible to evaluate the same treatment for several diseases (basket), several treatments for the same disease (umbrella), or several treatments and diseases in an adaptive way (platform)1. The master protocol defines the general framework of the study: objectives, methodology, evaluation criteria, analysis process. It does not include indication- or product-specific details. These elements are included in independent sub-protocols, each corresponding to a targeted sub-study. Each sub-protocol can be developed, submitted to an ethics committee and implemented more rapidly, as it fits into an already approved structure.
A better-recognized executive since 2022.
The approach gained recognition when, in March 2022, the FDA published an official guideline on the use of these protocols in n oncology studies3. This document provides recommendations on design, safety, regulatory aspects and interactions with the FDA to ensure well-structured, safe and effective trials capable of supporting marketing authorization applications. Following on from this, in May 2022, the EMA published a document entitled " Questions & Answers on Complex Clinical Trials (CCT) ", in which it explicitly addresses the notion of master protocol4. The ICH5 aims to harmonize protocol formats (e Protocol Template via ICH M11) and promote trial structuring, thus providing a favorable framework for modular master protocol approaches6.
The advantages of master protocol for medical devices.
The entry into force of Regulation (EU) 2017/745 (MDR) has established a rigorous framework for demonstrating the safety and performance of a medical device, both before and after marketing.
In this context, the master protocol approach offers several advantages:
- Pooling of resources: sites, personnel, data collection infrastructure (electronic data capture).
- Methodological agility: ease of adding new products or new indications due to a pre-existing framework described in the master protocol.
- Data consistency: sub-studies follow a harmonized framework, facilitating comparisons and meta-analyses. This last point is very important in order to capitalize on all the data collected for a particular pathology, product range, etc.
Two examples of master protocols in cardiology and oncology
The use of framework protocols is still fairly anecdotal in the medical devices sector. There are, however, a few convincing examples. MANTRA is an international post-marketing study (PMCF) using a master protocol dedicated to three valve devices: aortic, mitral and tricuspid. The aim of this investigation is to monitor performance and safety at 30 days and over the long term. The master protocol concept offers the manufacturer/sponsor a unique scalable framework for adding new sub-studies as required, and for providing post-market clinical follow-up information on the entire heart valve portfolio in a common database.
In their review, Bitterman et al. describe a phase I and II master protocol to evaluate the use of stereotactic adaptive radiotherapy guided by Magnetic Resonance Imaging (MRI), on cancers of different locations: lung, kidney, pancreas7. This review highlights the specific case ofRadiation Oncology Devices(RODs), which are difficult to evaluate clinically due to several constraints: heterogeneity of patients and treatments, strong dependence on the operator (e.g. technician, radiotherapist), and reduced budgets to fund trials. To overcome these limitations, the authors recommend the use of master protocols as a lever for regulatory, financial, logistical and methodological efficiency.
An approach better adapted to current regulatory requirements
In conclusion, master protocols represent a strategic opportunity for clinical research on medical devices. To maximize their benefits, it is advisable to plan a modular, scalable architecture from the outset: each sub-protocol must be able to be modified without impacting the whole study, and data must remain segmentable for each arm. The gradual adoption of such an approach by manufacturers paves the way for more agile, better harmonized clinical development programs, more closely aligned with current clinical evidence requirements.
1 Woodcock et al. N Engl J Med 2017;377:62-70. DOI: 10.1056/ NEJMra151006
2 Recommendations for planning and conducting clinical trials with master protocol designs: Umbrella, Basket and Platform Trials. ERA4Health booklets. July 2025
3 Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics Guidance for Industry-Guidance for Industry- March 2022
4 EMA - Complex clinical trials - Questions and answers Version2022-05-23
5 ICH : International Council for Harmonisation of Technical Requirements


