TELMIS, votre outil de gestion des essais cliniques contrôlés
TELMIS est la solution CTMS conçue pour offrir aux acteurs de la recherche clinique une gestion centralisée, structurée et conforme de leurs études. De la planification à la coordination des centres, elle permet de gagner en efficacité tout en sécurisant chaque étape du processus
Développée par des professionnels du secteur, la plateforme répond aux exigences opérationnelles des promoteurs académiques, industriels et institutionnels, en intégrant les standards réglementaires et les meilleures pratiques de terrain.
Avec TELMIS, vous pourrez mener efficacement vos études cliniques et vous concentrer sur l’essentiel : la qualité et la réussite de vos projets.
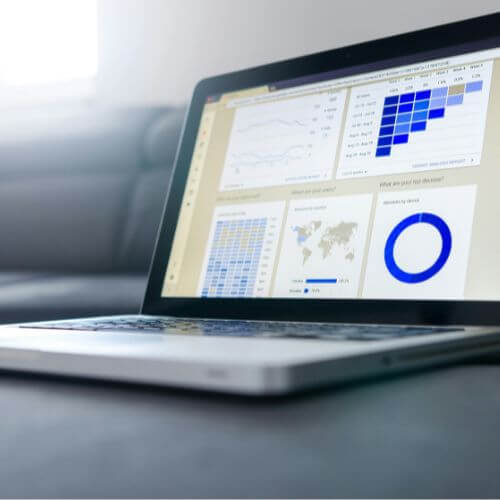
- Planification et suivi en temps réel : son interface permet de suivre les inclusions, les visites ou les évènements indésirables. Avec des tableaux de bord personnalisés, vous gardez une vue globale et actualisée de vos études.
- Gestion des centres, contrats et documents : TTELMIS centralise toutes les données liées aux centres investigateurs pour offrir un pilotage fluide et sécurisé ;
- Coordination des équipes : les utilisateurs disposent d’un accès personnalisé et d’une traçabilité des actions pour faciliter la gestion de leurs activités.

- Gain de temps : en réduisant les charges administratives et en automatisant les processus, TELMIS libère du temps aux équipes de recherche afin de pouvoir se concentrer sur la coordination et la qualité ;
- Contrôle qualité et conformité : il renforce la qualité des études et facilite la traçabilité documentaire avec des données exploitables à tout moment ;
- Accompagnement humain : avec TELMIS, bénéficiez de bien plus qu’un simple outil. C’est également toute une équipe projet dédiée, réactive et à l’écoute.
TELMIS, l’outil de gestion des études cliniques en toute maîtrise
.
Nos engagements en matière de qualité


