TELMIS, your tool for managing controlled clinical trials
TELMIS is the CTMS solution designed to offer clinical research players centralized, structured and compliant management of their studies. From planning to coordinating centers, it increases efficiency while securing every stage of the process.
Developed by industry professionals, the platform meets the operational requirements of academic, industrial and institutional sponsors, integrating regulatory standards and best practices in the field.
With TELMIS, you'll be able to conduct your clinical studies efficiently and focus on what's most important: the quality and success of your projects.
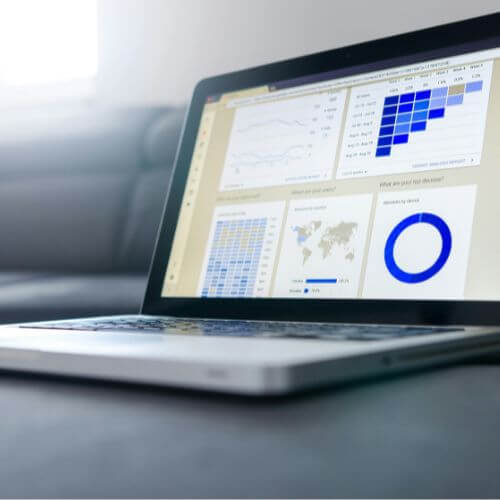
- Planning and monitoring in real time: its interface enables you to track inclusions, visits or adverse events. Customized dashboards give you a global, up-to-date view of your studies.
- Center management, contracts and documents: TTELMIS centralizes all data related to investigator centers to provide fluid, secure management;
- Team coordination: users have personalized access and traceability of actions to help them manage their activities.

- Saves time: by reducing administrative burdens and automating processes, TELMIS frees up time for research teams to concentrate on coordination and quality;
- Quality control and compliance: reinforces the quality of studies and facilitates document traceability with data that can be used at any time;
- Human support: with TELMIS, you benefit from much more than just a tool. It's also a dedicated, responsive project team.
TELMIS, the ultimate clinical trial management tool
.
Our quality commitments


