Distantia facilitates the collection, remote monitoring
and analysis of clinical data at a lower cost
Distantia is a solution developed for sponsors, CRAs and investigators to monitor source data remotely, while complying with regulatory requirements (GCP, GDPR). It is now possible to monitor data in real time, carry out remotesource data verifications (rSDV) and facilitate communication between theinvolved parties.
At a time when quality, responsiveness and safety are more than ever at the heart of the challenges facing clinical research, Distantia is an innovative digital platform that adds an unprecedented dimension to your studies, while optimising costs and timescales.
To ensure efficient and reliable study management, the solution offers powerful features designed to simplify daily operations and enhance data quality.
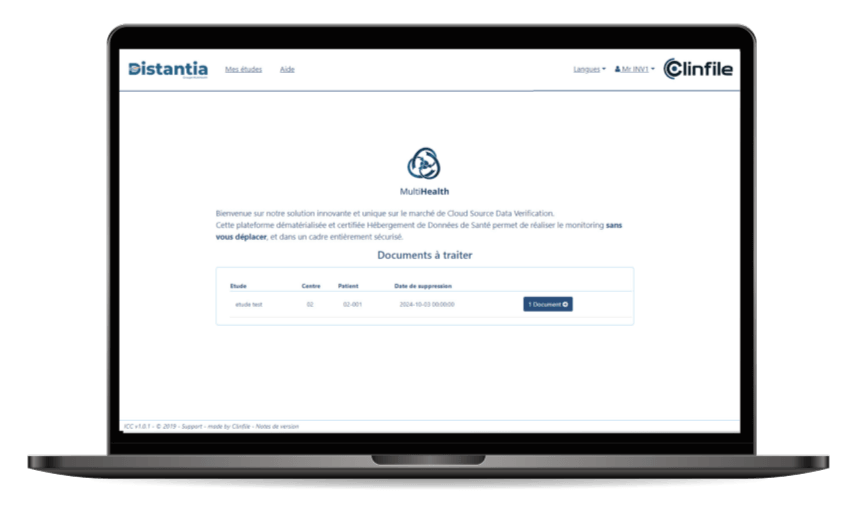
- Customisable e-CRF: its intuitive interface makes it easy to create forms. The e-CRF can be put online quickly, so you can deploy your studies efficiently;
- Real-time monitoring follow-up: dynamic dashboards, interactive and historical queries for effective management and traceability;
- Exports, interoperability and archiving: data export, connection to other tools and certified archiving make this tool indispensable.
These advanced functions give research teams greater control, flexibility and security throughout the study lifecycle.
Our solution is designed to simplify clinical research while ensuring data quality and strong human support at every stage.

- Time saving: rapid deployment and remote monitoring mean you can focus on conducting the study, reducing manual tasks, data entry errors and set-up times;
- Continuous improvement: consistency checks, alerts and forms guarantee reliable data collection, for a high-quality database;
- Human support: Distantia relies on a dedicated project team, from design to training and technical support.
This support-driven approach ensures that each study runs smoothly, efficiently and with full confidence.
Our quality commitments


